Process validation consists of three stages that are interlinked to each other.
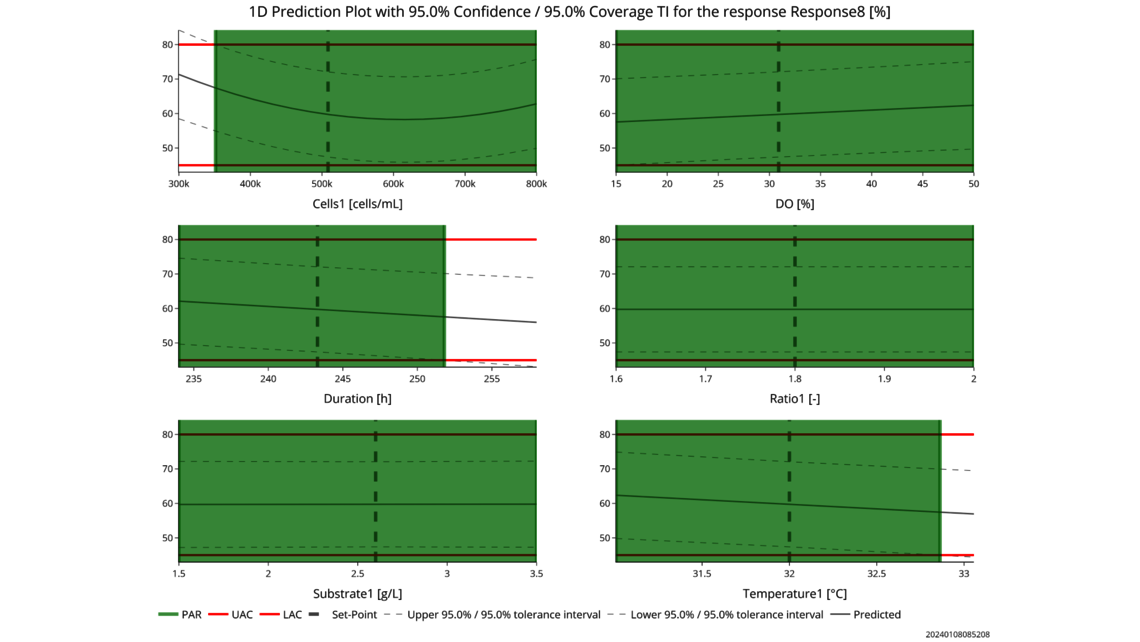
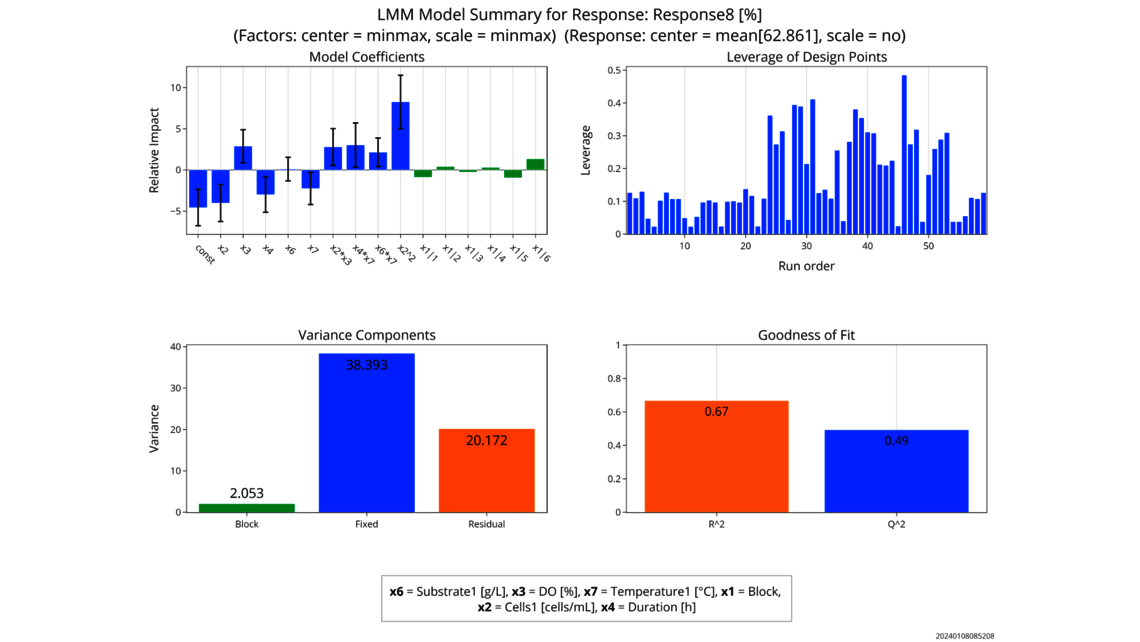
The first one, Process Characterization, is the most essential step to establish a Control Strategy in accordance with FDA, EMA and ICH guidelines, ensuring consistent product quality. To achieve this activity, the relationship between process inputs (process parameters, material attributes) and the process output (critical quality attributes, product amount) needs to be understood and controlled. This relationship can be learned from data and prior knowledge.
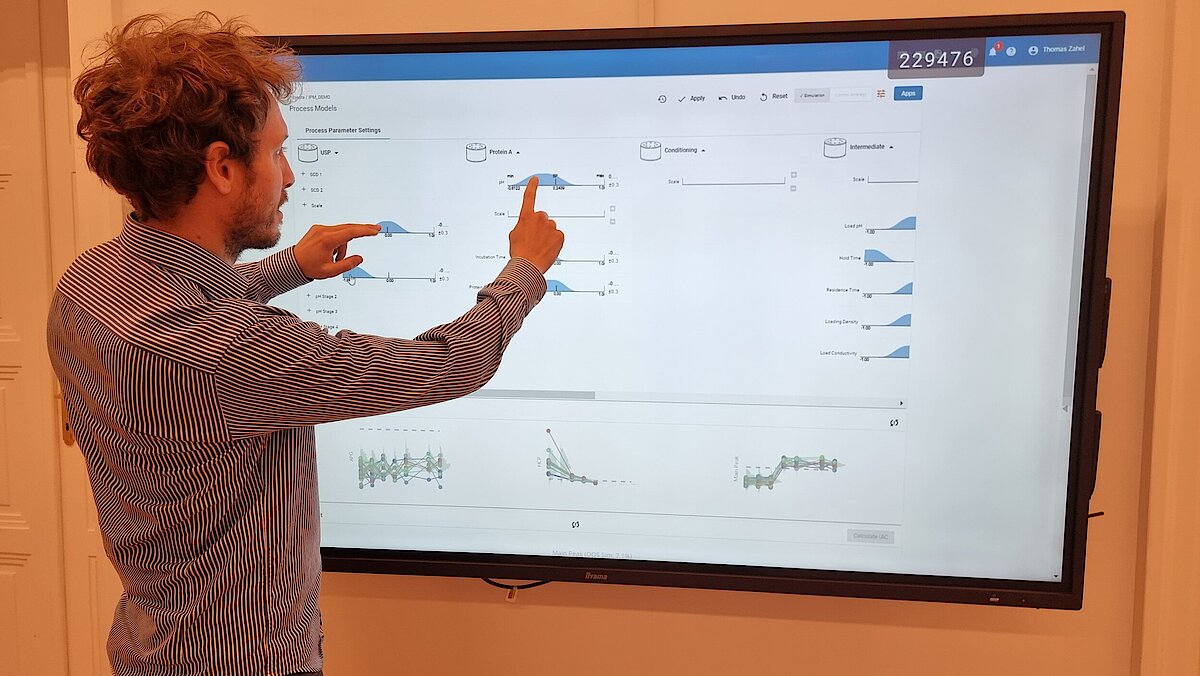
Our unique workflow guides you through this critical phase, comprising:
- Risk assessment
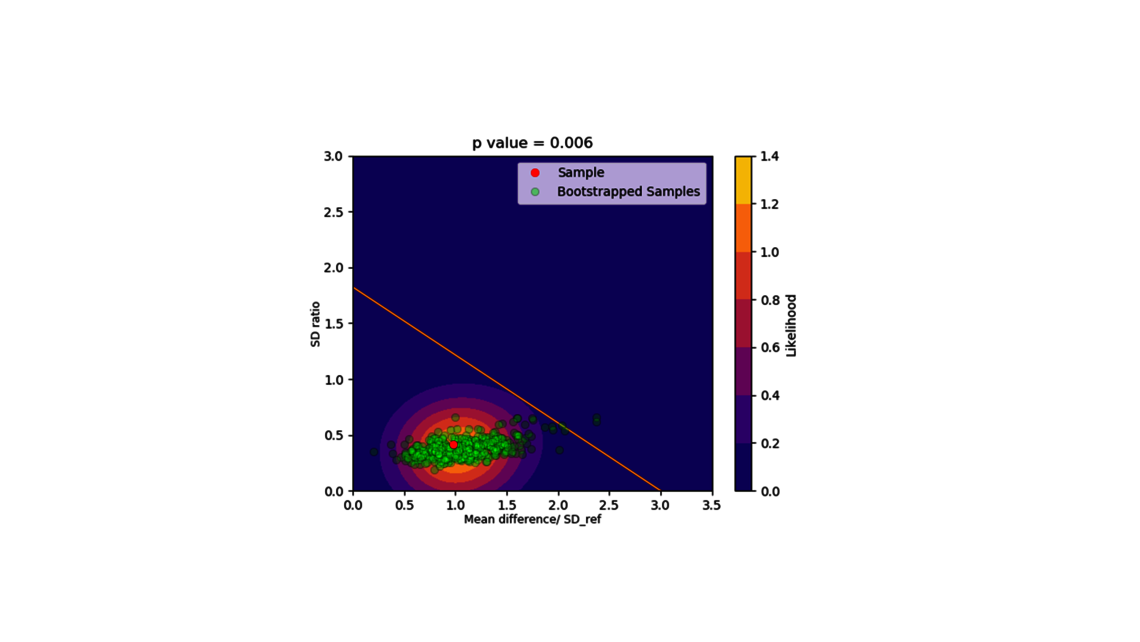
- Scale down model establishment and comparison
- Experimental design (for statistical and dynamic models)
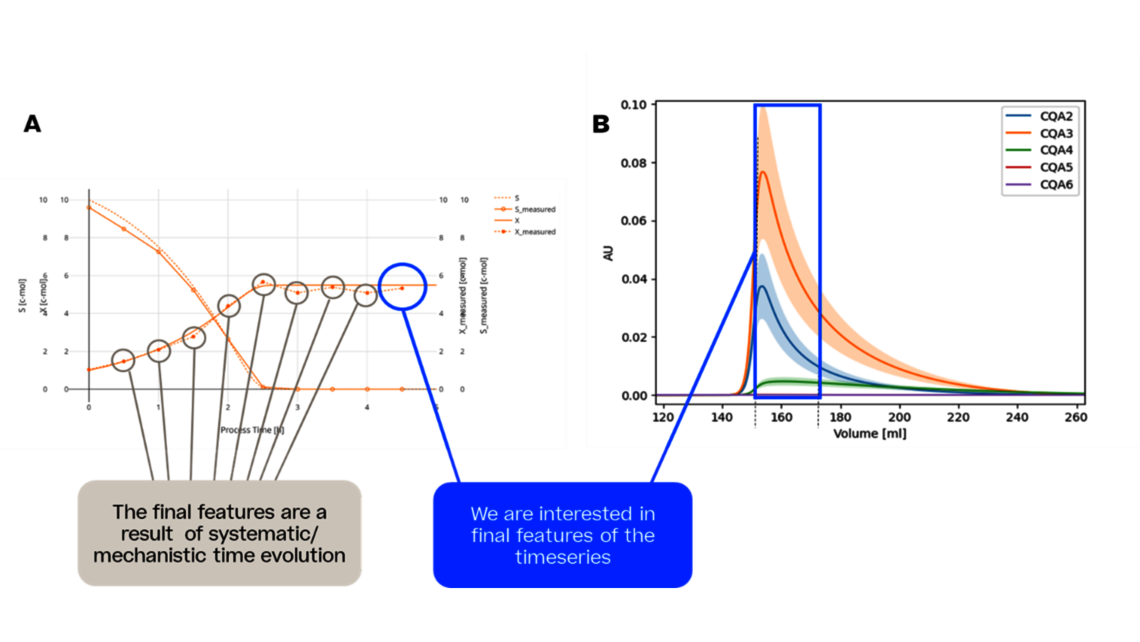
- Experimental analysis and criticality assessment (linear regression models, linear mixed models including random effects, dynamic models for up- and downstream)
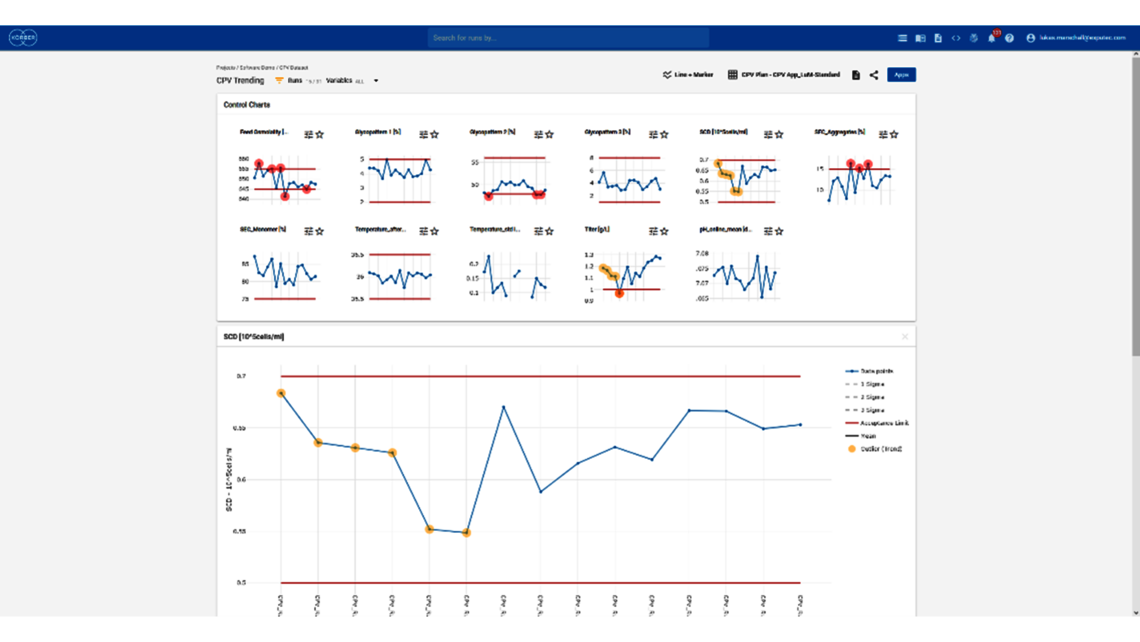
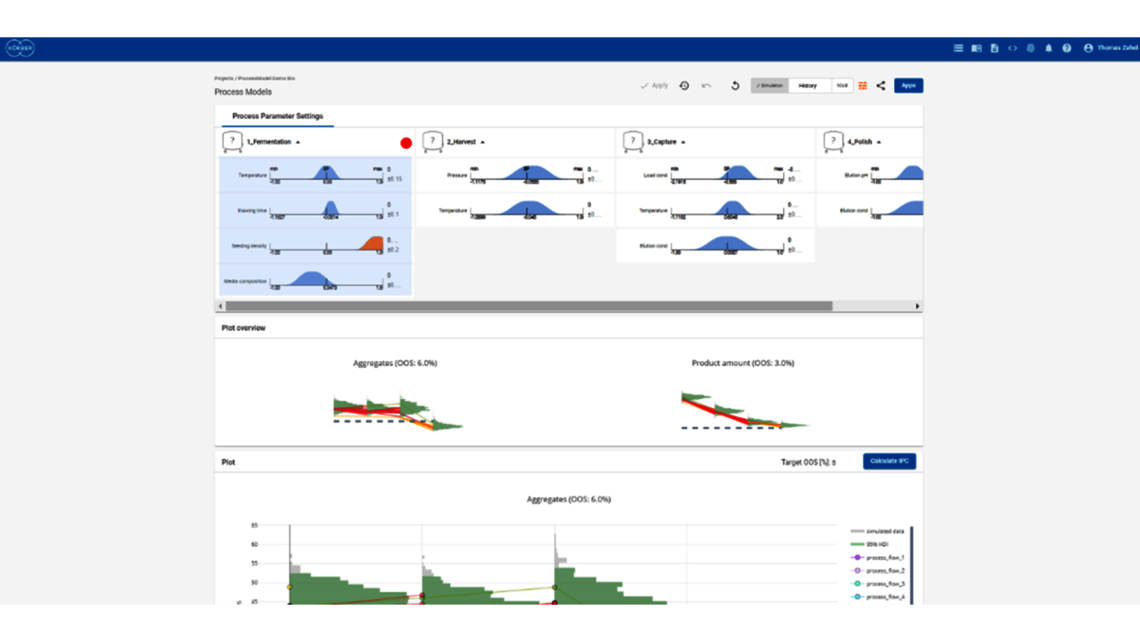
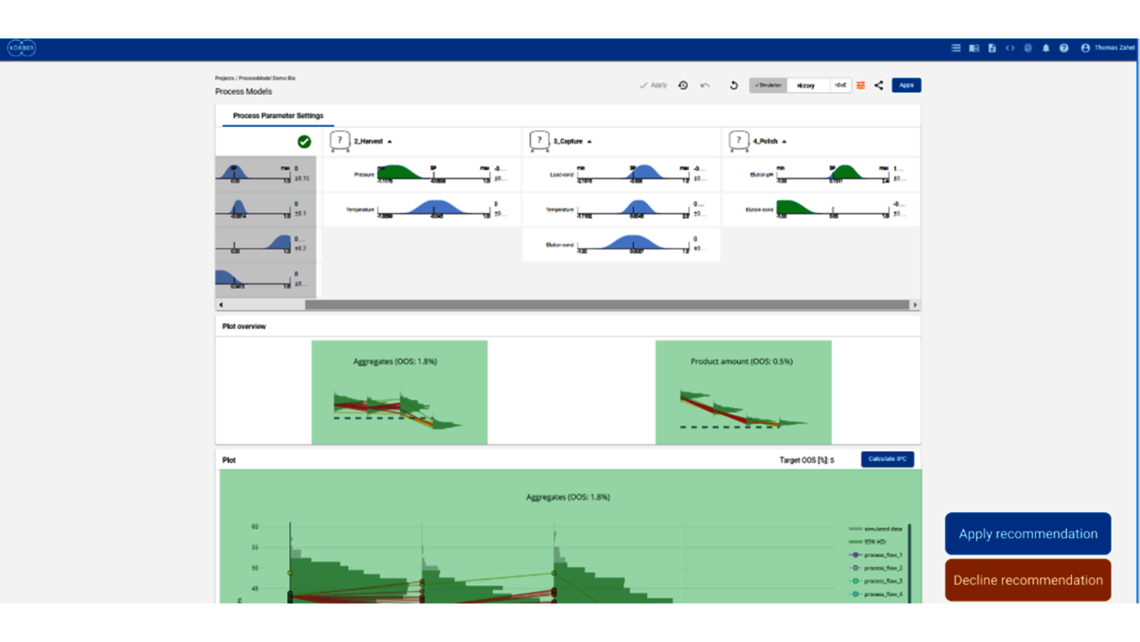
- Setting a holistic control strategy using the end-to-end process modelling framework
With over a decade of experience and involvement in more than 20 product commercialization, we've fostered productive exchanges with regulatory authorities and have gained expertise across diverse product areas like monoclonal antibodies, recombinant protein, pDNA, and more.
We have published over 10 peer reviewed papers with large pharmaceutical companies like Boehringer Ingelheim and Takeda that demonstrate the effectiveness of our approach. In a recent peer-reviewed scientific publication, we demonstrated that our method results in a reduction of over 50% in the number of experiments required by leveraging self-learning digital shadows. This method was also demonstrated at many conferences like the 2023 ISPE Biotechnology conference.
We support you with:
- Faster time-to-market by reducing number of experiments by >50%
- Regulatory accepted workflows and risk assessments according to ICH Q9 / ICH Q12
- Holistic control strategies leading along ICH Q8 to widest possible PARs and design spaces
- Data based criticality assessment of process parameters
- Timely delivery of results