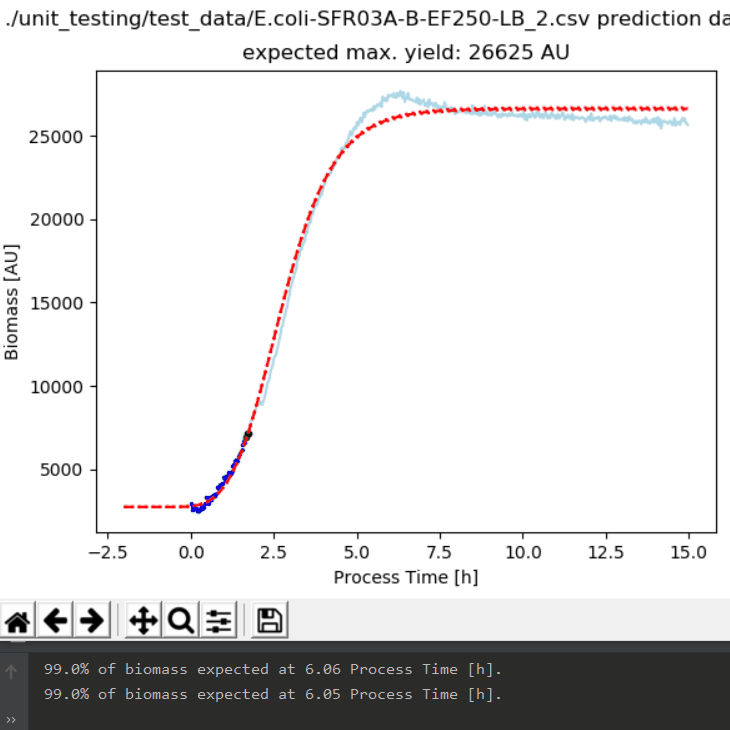
One of the main challenges in pharmaceutical manufacturing is how to quickly characterize a new strain or clone for robust bioprocessing along the product life cycle. Process development groups in industry as well as in academia share a common task: Strains and clones are quickly generated by molecular biology. However, it remains a major question which one to choose for developing a robust process and how to avoid failures during scale up and along the product life cycle. A further challenge is that proper strain/clone characterization shall be based on strain/clone-specific physiological parameters (Key Performance Indicators, KPIs) that describe the strain/clone independent of scale and initial conditions.
An ideal system would be an integrated experimental setup allowing for easy experimentation, representative sampling and proper analytics as well as objective data analysis to derive strain/clone specific physiological parameters. To develop such a system, experts from academics (Zurich University of Applied Sciences/ZHAW) and industry (PreSens, Exputec – now part of Körber’s Business Area Pharma) partnered in a unique collaboration with a promising result.