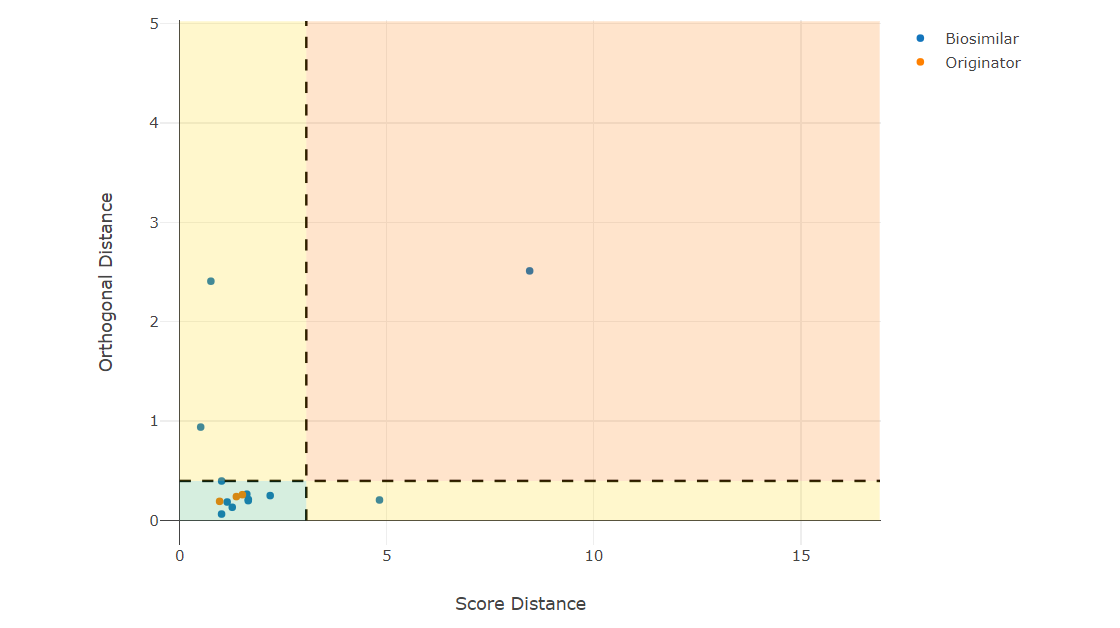
How should I proceed during biosimilar screening to detect biosimilarity?
As a biosimilar developer, you first have to screen for a production clone that produces a product similar to the originator. Typically, you measure multiple critical quality attributes of the product. To be cost-efficient, you typically compare the analytical results of one strain to the originator population. It is highly challenging to perform inferential statistics in this situation since you have only one measurement of the clone and need to conclude for that. This follows the EMA reflection paper, which states that inferential statistics is not always feasible, especially when comparing single values to a population.
Especially for biosimilar screenings, multiple CQAs are measured, which are correlated with each other. Hence, it is good to use multivariate methods that have increased power to detect similarities and potential differences.