Projecting digitalization to the bioprocess industry
The bioprocess industry is currently being transformed and disrupted through the capabilities of digitalization and the Internet of Things (IoT), as summarized under the buzzword Industry 4.0. Digitalization in the bioprocess industry creates new forms of innovation and results in new business models: The bioprocess industry is about to go through a revolution by taking full advantage of what innovative digital technologies offer. Industry 4.0 is present in all aspects of the bioprocess industry, as it will have an impact throughout its value chains, from logistics, process- and materials design, planning, plant operations, plant safety, monitoring and maintenance of factory equipment to marketing/sales and supplier/customer integration. This has been nicely summarized in a recent DECHEMA white paper.
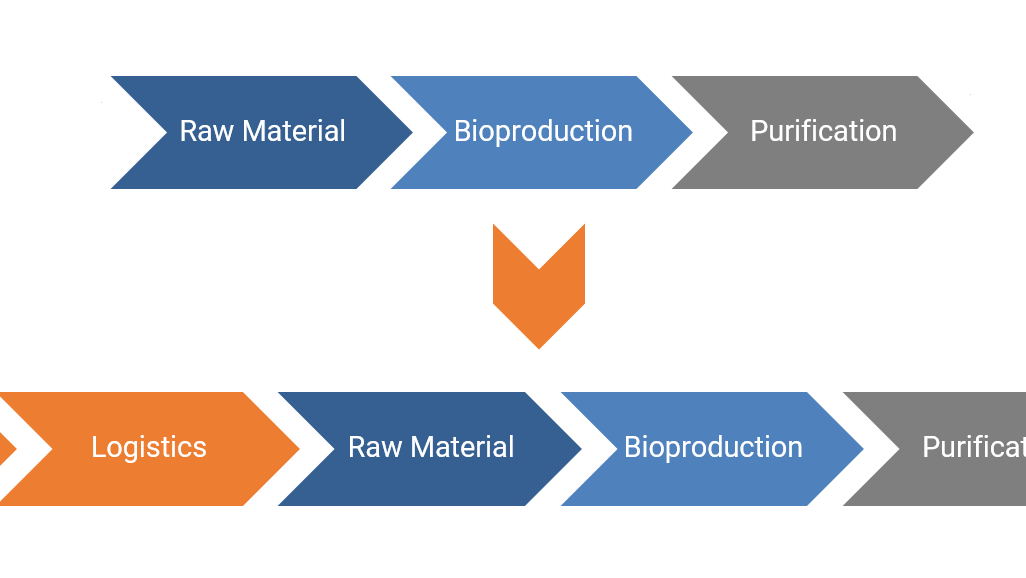
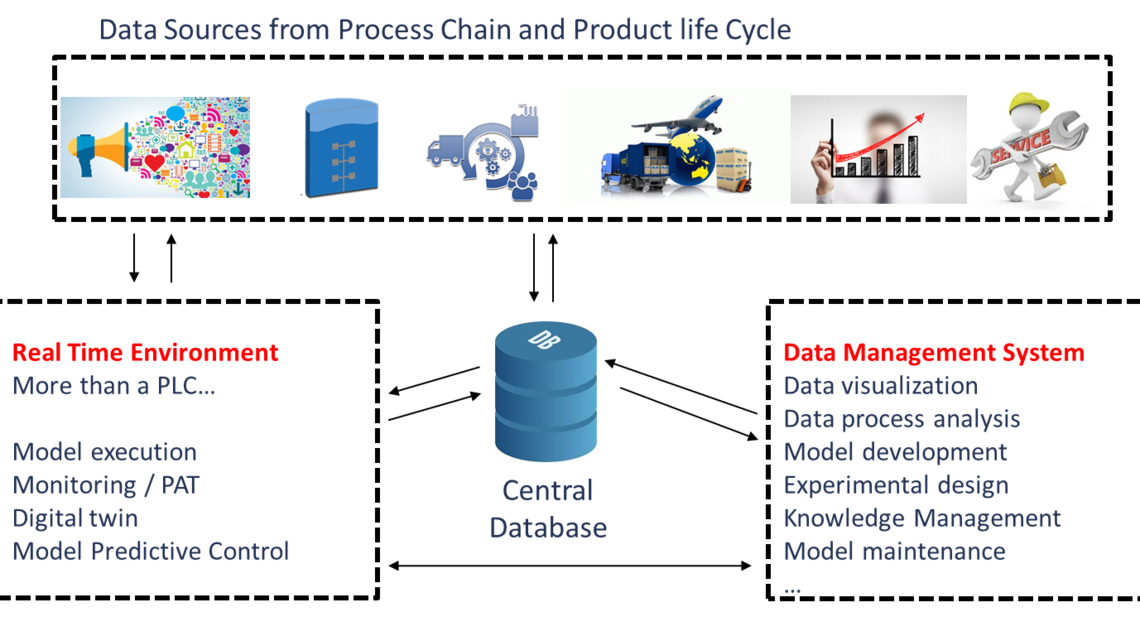
In more detail, digitalization for the bioprocess industry will mainly act on two dimensions: A) the process chain and B) the product life cycle.