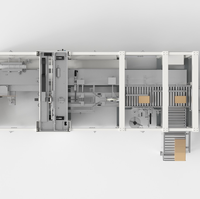
The need to digitize manufacturing industries stems from the growing push for interconnectivity between different devices within an existing internet infrastructure. The Industrial Internet of Things (IIoT) or digitization trend can be subdivided into various trends such as sensorization, predictive analytics, artificial intelligence (AI), and cloud computing.
These trends offer many advantages, including the automatic detection of issues led by auto triggers in case of any abnormality in the manufacturing facility. They also help transform manufacturing tasks like the visual inspection of equipment in a plant, including quality checks to meet regulatory guidelines. Secure data storage, aided by cloud-based servers, can manage a variety of operations, ranging from enterprise resource planning (ERP) and financial management to data analytics and workforce training. Finally, IIoT ensures proper integration of supply chains, which is one of the major requirements of the manufacturing industry.
The goal of these technologies is to help industrial manufacturers manage their businesses with better intelligence and to make the manufacturing units more productive, cost- and energy-efficient, safe and, most importantly, streamlined.