As a result of that holistic approach, the control strategy stays rigid where this is required to reach drug substance specifications. But it is as wide as possible to obtain manufacturing flexibility.
Executive Summary
A successful PCS study is key to reduce time-to-market of any biopharmaceutical product. This can be achieved by the unique workflow established by the experienced consultants from Körber:
- A process validation master plan ensures that scope, deliverables, and timelines are aligned between all stakeholders of a PCS.
- Statistical occurrence analysis and new risk rating scales reduce subjectivity during risk assessments.
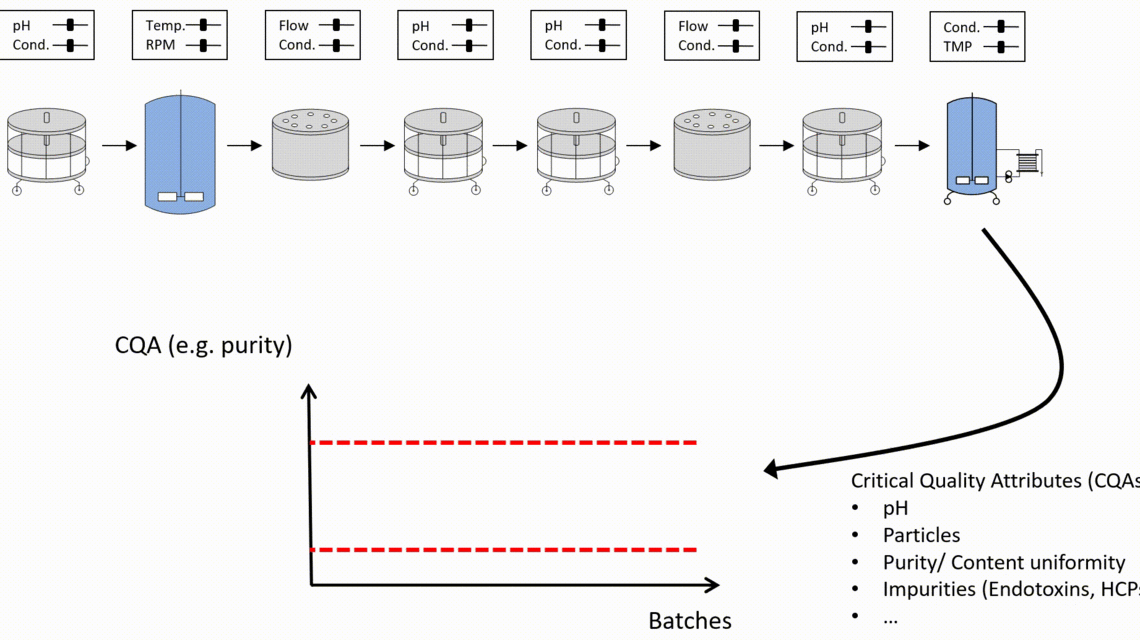
- Innovative visualization techniques ensure fast and precise identification of critical unit operations to reach the required Quality Target Product Profile. Understanding how impurities are created and cleared in your process helps to plan the right type of experiments, i.e., spiking studies vs. DoE studies.
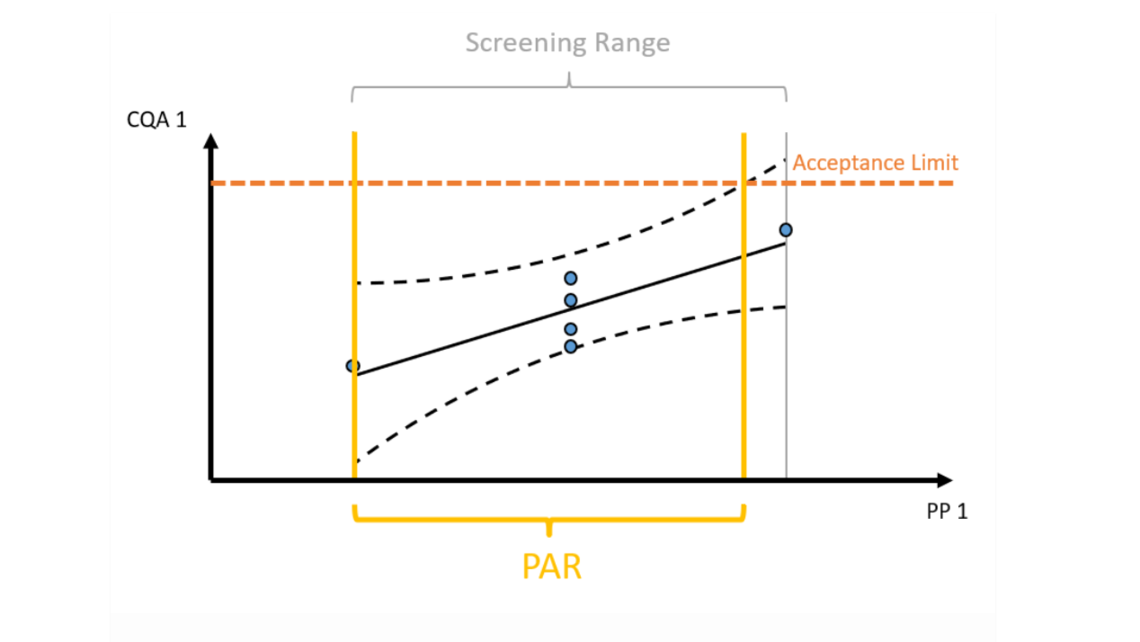
- Statistical equivalence testing ensures not to overlook practically relevant differences between scales. These offsets need to be considered for predictions at manufacturing scale.
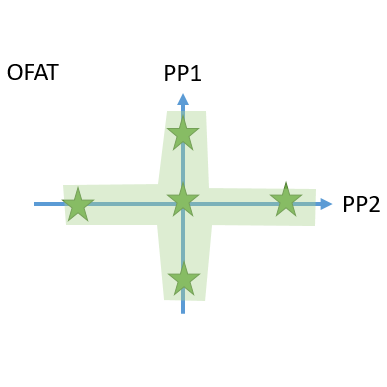
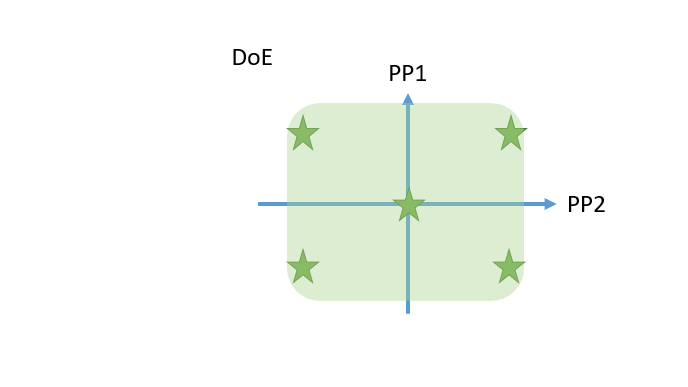
- Cutting edge optimal experimental designs enable to identify main and interaction effects of PPs onto CQAs with a minimum number of experiments.
- Establishment of statistically and practically relevant models enables the establishment of integrated process models.
- Only holistic definition of the control strategy using integrated process modeling ensures meeting drug substance specifications and thereby ensures patient’s safety.
Planning, analysis, and execution of necessary experiments is at the center of the PCS work. Statistical tools such as data assisted risk assessment, DoE planning and integrated process modeling can facilitate this work and ensure the right focus and reduction of experimental effort, setting of feasible control strategies for manufacturers and finally timely market entry.
With PAS-X CMC Consulting, Körber assists with in-depth knowledge from its combined experience in data science and pharmaceutical production processes. Our consultants have 8+ years of experience in consulting and statistical services for process characterization and validation and have successfully contributed to bringing 20+ products to the market.
Get into contact with our experts on any related questions or for detailed information on what we can provide.
Contact us