HIGHLIGHTS
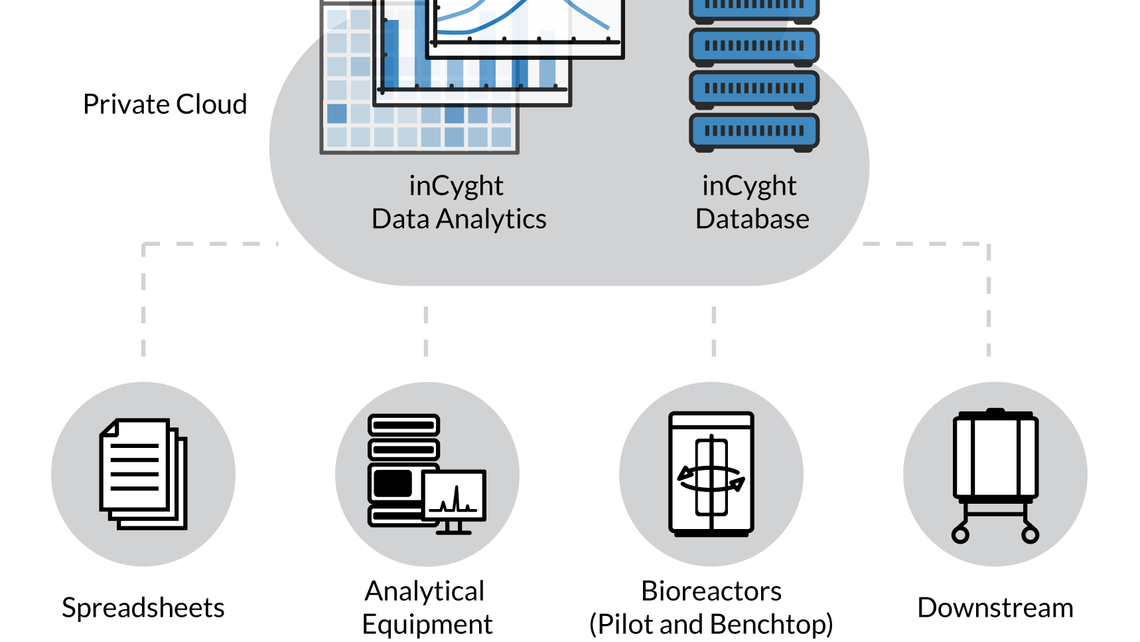
- INTRAVACC is implementing the Werum PAS-X Savvy platform for data management, (real-time) data analytics, multivariate statistics, and mechanistic modeling.
- All data from upstream and downstream processes, bioreactors, spreadsheets, and analytical equipment is stored in a central database.
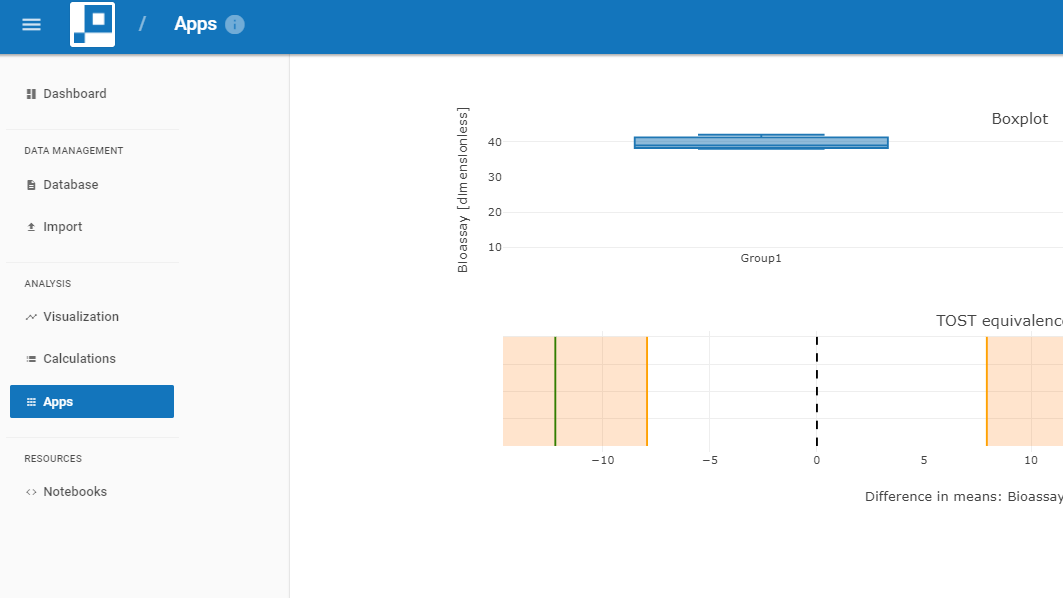
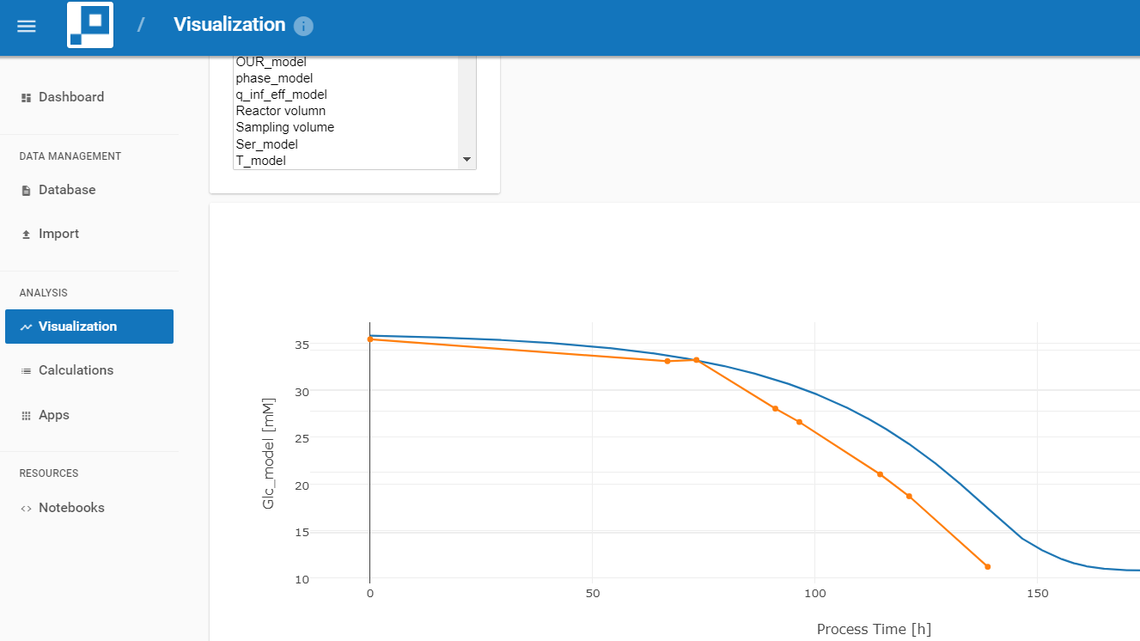
- Intravacc scientists run visual bioprocess data analytics on real-time data within the web browser.
Bioprocess development generates extensive data (from in silico or in vitro experiments) that must be analyzed using a properly structured methodology. This data can be generated from diverse sources, and it can include large datasets (e.g., time series, pictures, quality measurements, etc.). A data logging and data analysis strategy must be clearly defined and adequately executed to take full advantage of the experimental work. Bioprocess development can be accelerated only after having such a strategy, which would allow maximizing the findings from each experiment.
Intravacc (the Institute of translational vaccinology) is currently developing its strategy for data logging and analysis with the assistance of PAS-X Savvy. This permits Intravacc to continue as one of the leading research centers in vaccine technology.