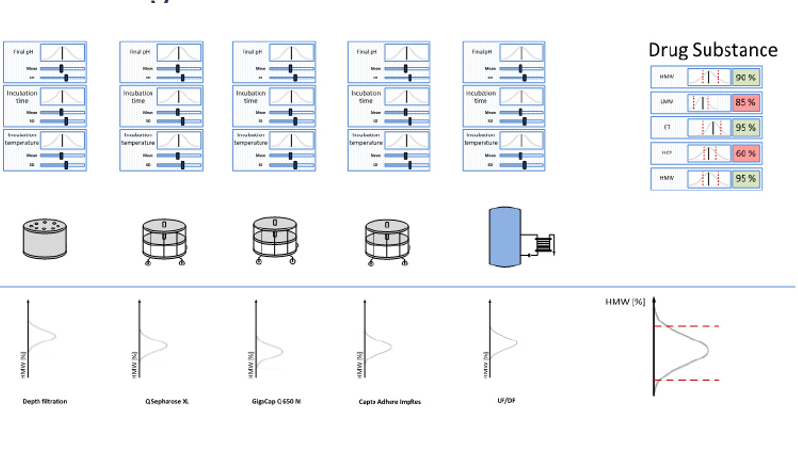
Bioprocess digital twins are an exciting new technology that changes how we do bioprocess development, process validation, and cGMP operations.
In 2016, Colin Harris, VP of Software Research at GE, presented a tool for industrial processes that was positively fascinating — science fiction turned into reality. Colin Harris interacted with a voice-activated software to remotely mitigate damages to a wind turbine on the other side of the world. He addressed the software as “Twin,” and the two calmly saved millions of dollars within seconds. This is the future, right?
Yes! Digitalization. Artificial intelligence. Data Analytics. There is a new tech wave in the process industry. Digital twins are becoming a reality in many industries. Bioprocessing is no exception.
This article delves into the “Bioprocess Digital Twin” technology for bioprocessing and explores how it is changing the way we do bioprocess development, process validation, and GMP manufacturing.