Beschleunigen Sie Ihre Prozesse in der Produktentwicklung, Herstellung, Validierung und im Tech-Transfer. Mit Werum PAS-X Savvy von Körber managen, visualisieren und analysieren Sie Ihre bioprozesstechnologischen Daten in Echtzeit.
Sie wollen agil, sicher und robust von der Entwicklung bis zur kommerziellen Produktion gelangen? Nutzen Sie das verborgene Potenzial Ihrer Prozessdaten.
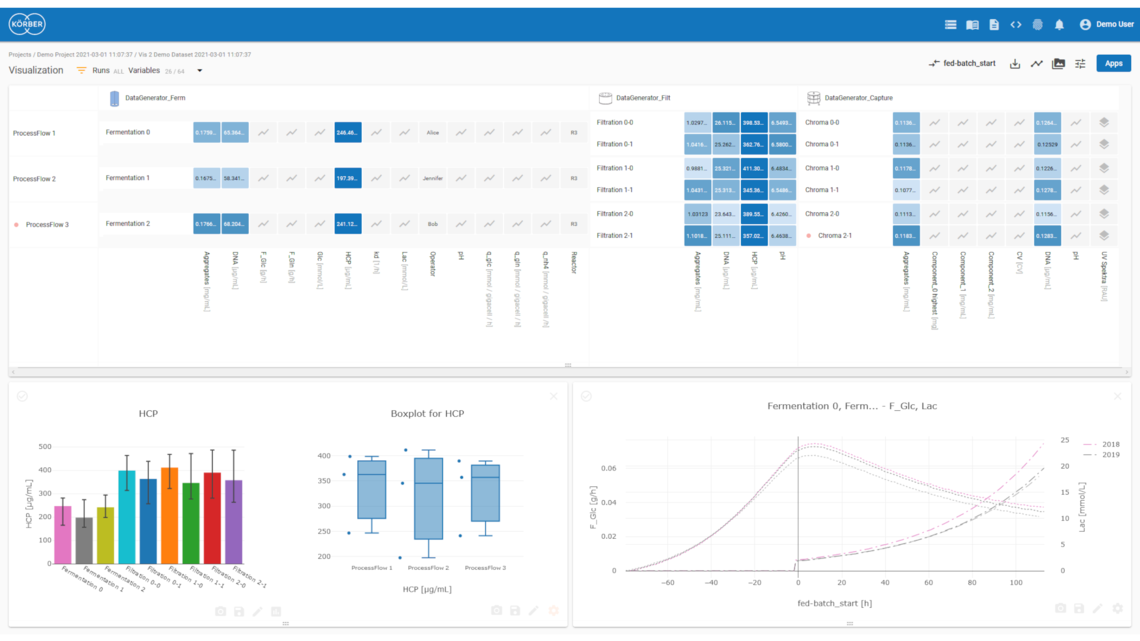
Werum PAS-X Savvy kombiniert nahtloses Datenmanagement, benutzerfreundliche Datenvisualisierung, ganzheitliche Datenanalyse und automatisierte Ergebnisberichte in einer kollaborativen Analyseplattform. Mit unserer Lösung managen, visualisieren und analysieren Sie Ihre Daten aus allen Phasen des Produktlebenszyklus und werten diese automatisiert für Ihre Berichte aus.